For Providers
Your Partner in Preventing Diabetic Limb Loss
Supporting Foot Health Through Proactive Monitoring
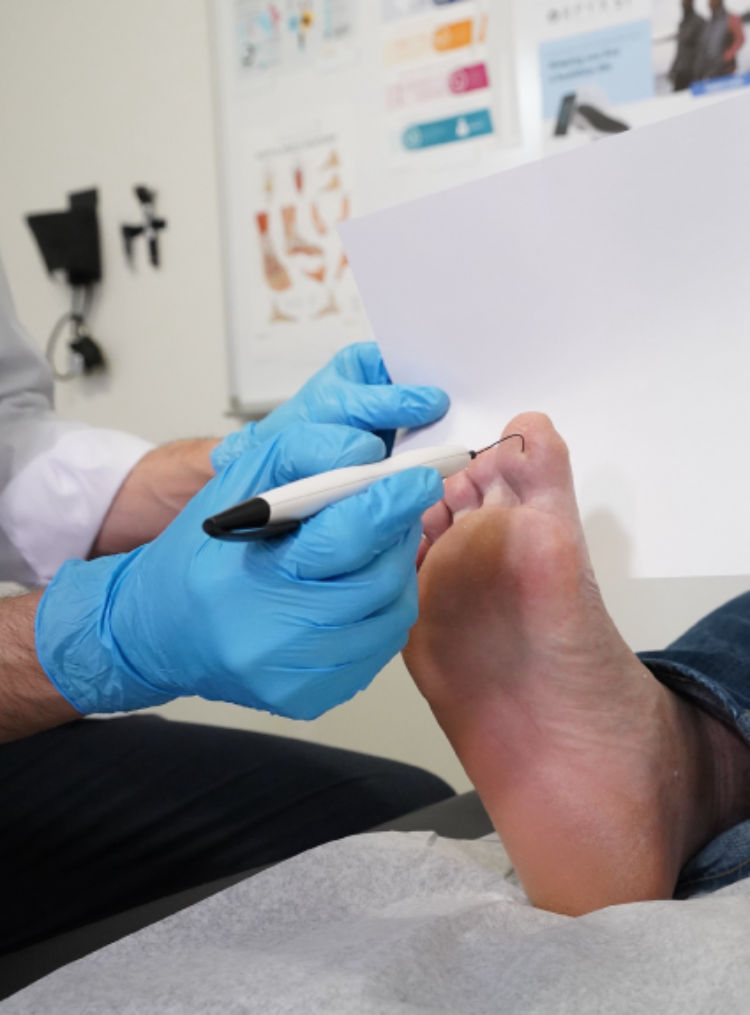
At Orpyx®, we are committed to improving foot health for individuals living with diabetes. We are focused on helping to prevent diabetic foot complications, including limb loss, through early detection, continuous monitoring, and proactive care.
The Orpyx Sensory Insole program combines advanced sensory insoles with remote patient monitoring to support proactive care. The system continuously monitors plantar pressure, temperature, and activity data, while our US-based nursing team reviews trends and supports patient adherence.
For patients, the system is simple and doesn't require charging. For the provider, we deliver trusted support that keeps you and your patient connected between medical visits.
A Shared Vision for Ending Limb Loss
The Only Plantar RPM Solution Backed by a Randomized Controlled Trial¹
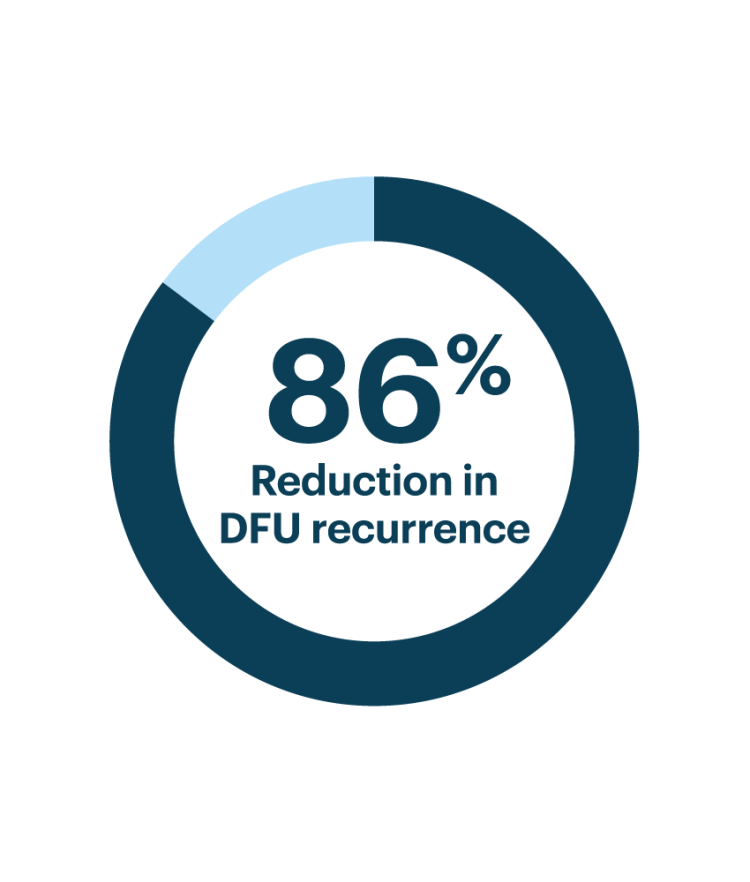
In a randomized controlled trial (RCT) of patients with diabetes and a history of foot ulcers, the Orpyx technology, delivering real-time pressure alerts, demonstrated a substantial reduction in ulcer recurrence.
Among patients who wore the insoles for at least 4.5 hours per day, ulcer incidence was reduced by 86% compared to standard care.¹
This highlights the critical role of consistent use and real-time data in supporting early intervention and long-term limb preservation.
Empowering People. Supporting Providers.
Our Between Visit Services provides trusted support that keeps you and your patient connected between visits, because your care doesn’t stop when the appointment ends.

Ongoing Personalized Coaching
Orpyx US-based nurses support the users foot care plan and help them make healthy changes that fit their lifestyle.
Flexible Communication Options
Every user is assigned a remote nurse for personalized support via phone or text.
Proactive Behavior Modification
Our remote nurses spot concerns early and guide changes to help prevent foot complications and avoid more serious interventions.
Scheduled and As-Needed Calls
Our remote nurses conduct monthly wellness calls to monitor and identify early foot health concerns before escalation.
Minimal Escalation
Only 4% of cases are escalated to the provider, as nurses support patients through early intervention and education.2
Actionable Insights
Multimodal insole data offers actionable insights to help inform the users foot care plan.
Clinical Rounds
We host collaborative clinical rounds to interpret RPM data, leveraging informatics to uncover insights, guide decisions, and enhance outcomes and engagement.
Seamless, Secure Integration
Our program integrates seamlessly into your workflows, providing secure, encrypted, HIPAA-compliant data.
Real Experiences. Real Impact.
CPT® Code Considerations*
Initial set up and patient education on use of medical device
Supply of medical device with daily recordings and programmed alerts
1. Abbot CA, et al. Innovative intelligent insole system reduces diabetic foot ulcer recurrence at plantar sites: a prospective, randomised, proof-of-concept study. Lancet Digital Health Oct. 2019; 1: e308–18. (Study was conducted with first generation SurroSense Rx® which has the same pressure algorithm as the Orpyx technology) 2. Data on file—Escalations Analysis 2024. *The reimbursement information provided is intended to provide general information concerning coding of Orpyx products only. Orpyx does not guarantee coverage or payment for any products. The ultimate responsibility for proper coding, satisfying reimbursement requirements, and obtaining reimbursement remains with the provider. Coding and coverage policies and guidelines are complex, can vary from one carrier or region to another, and are updated frequently. Providers should check with their local carriers or intermediaries often and should consult with counsel, a reimbursement specialist for coding, coverage, or billing questions. Current Procedural Terminology (CPT®) is copyright by the American Medical Association. All rights reserved. CPT is a registered trademark of the American Medical Association (AMA). CPT codes and their descriptions do not reflect or guarantee coverage or payment.